What Researchers Say About the Treatment and the Management of Juvenile Dermatomyositis (JDM)
The diagnosis of Juvenile Dermatomyositis raises a lot of questions. Patients and families wonder, “how do I know the best treatment plan?”
A treatment plan is based on many factors, including the severity and expression of the JDM. Each case is different, and the symptoms can change over time. Some patients suffer more skin rashes, and others suffer from more muscle weakness. Physicians create treatment plans to address individual needs, but all plans are designed to provide the greatest benefit to the patient and minimize side effects.
Recently, some of the best physicians in the world who diagnose and treat JDM patients pooled their collective experience and published a series of consensus plans to standardize and optimize treatment for a variety of JDM patients. These plans can help you start discussing treatment options with your doctor. The plans are divided into categories, which are based on patient diagnosis.
1. Patients with new onset JDM, and patients diagnosed with JDM classified as “moderate.”
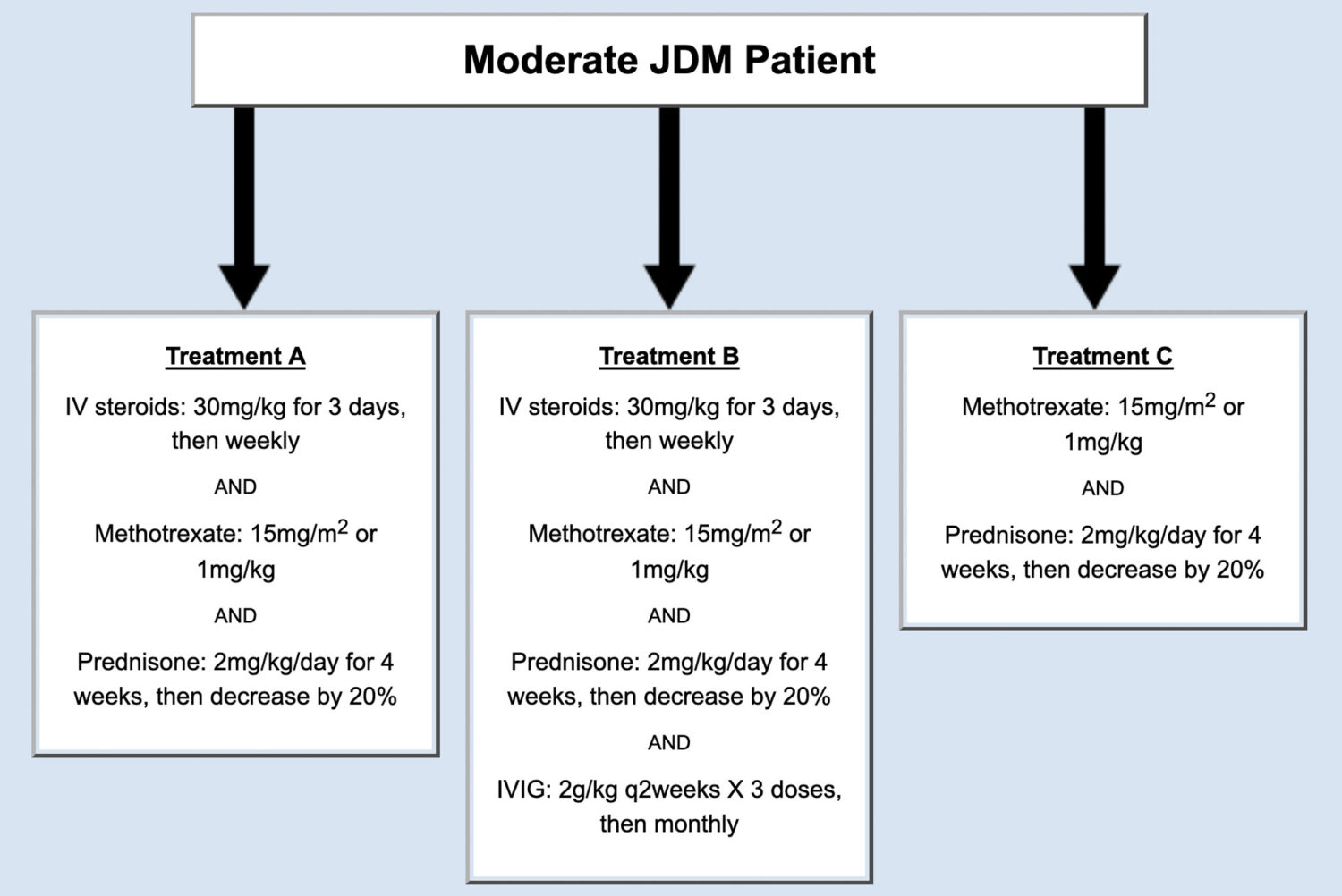
The panel of physicians agreed on three possible treatment plans for patients that met the criteria of moderate JDM (Figure 1).
The panel of physicians also created a consensus treatment plan for patients two-months after initial treatment.
This is a follow-up paper to the original, intended to describe consensus treatment plans for the same group of patients with moderate JDM beyond the first two months of treatment. Given the critical role of steroids in treating JDM, one of the key components of this work was consensus on a tapering regimen for steroids, balancing the risk of inadequately treating the JDM and the risk of side effects. This tapering was modified depending on whether the patient’s conditions was improved, unchanged, or worse. Some additional consensus was reached on further follow-up and investigations and is reported in this paper.
2. Patients with JDM who have improved symptoms of muscle weakness but have a continued skin rash.
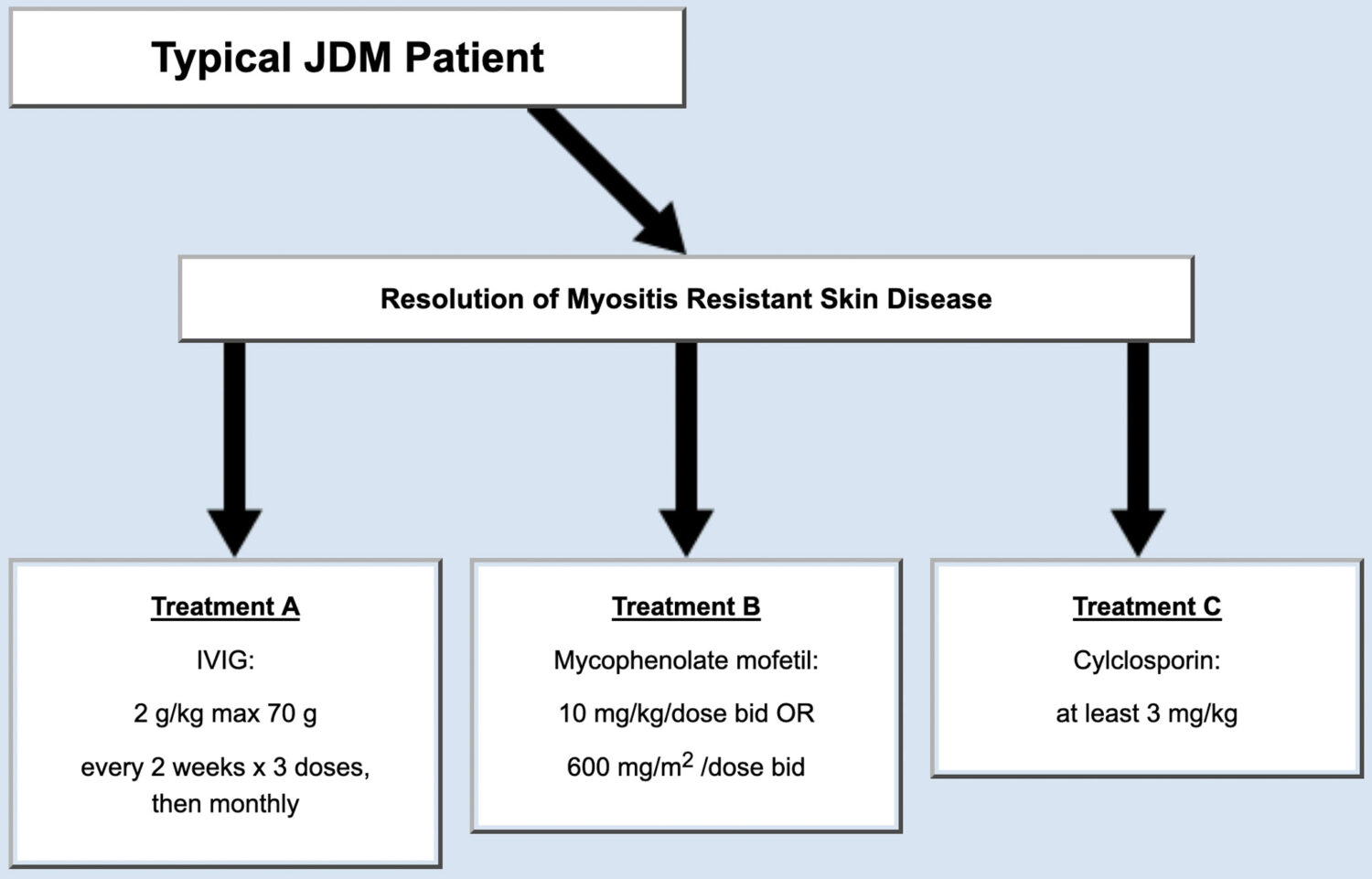
Following initial treatment, some JDM patients get much better. For example, some patients’ muscle weakness disappears; however, some symptoms persist, particularly a skin component of the disease. This subset of patients often requires changes to their treatment to improve their rash (Figure 2). For patients who met the criteria, the panel of physicians agreed on three possible treatment plans (Figure 2).
3. Patients with new onset JDM classified as amyopathic, skin predominant, or juvenile dermatomyositis sine myositis.
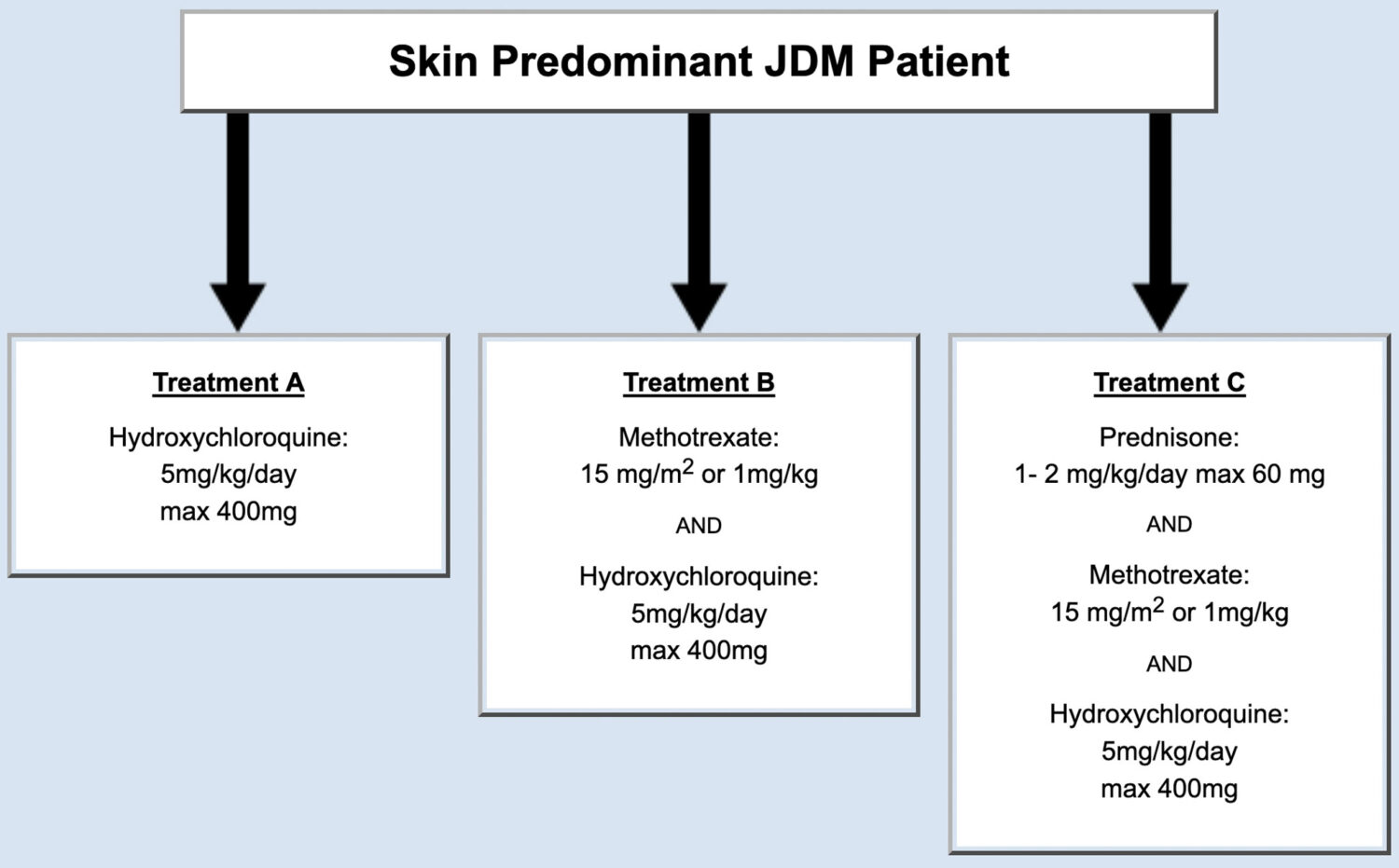
Some patients with JDM have a significant skin rash, but no significant weakness or muscle inflammation. Some physicians think this group of patients can be treated differently than those children with apparent muscle involvement (like weakness or abnormal tests like biopsy or MRI). The panel of JDM experts considered this subtype of JDM patients, developed consensus treatment plans, and published their findings (Figure 3).
The treatment plans recommended in this publication had a number of options, depending on the diagnosis and severity of the disease. Specific plans are to be chosen based on the the treating physician’s judgement.
4. European experience in management of JDM.
The consensus treatment plans you just read were completed by Childhood Arthritis and Rheumatology Research Alliance (CARRA). CARRA (carragroup.org), is a North American group of clinicians and researchers focused on the care of pediatric rheumatologic diseases. But, JDM is global, and groups outside of North America have made specific recommendations for the management of JDM. A European initiative called Single Hub and Access for pediatric Rheumatology in Europe (SHARE) have optimized and shared diagnostic and management plans for European children and young adults with JDM. The results of the SHARE initiative resulted in a peer-reviewed paper, which outlines specific, European approaches to care.
Since these recommendations were completed by and for European patient populations, it is still being determined whether the standards can work in North America.
It is our hope these plans will serve as a good starting point for conversations with your healthcare provider. The Cure JM Foundation is currently evaluating these consensus treatment plans and actively funding research programs to expand treatment options and the number of drugs used to treat and manage JDM.